Safety and Pharmacovigilance Certificate Program
DIA’s Safety and Pharmacovigilance Certificate Program is a competency-based program.
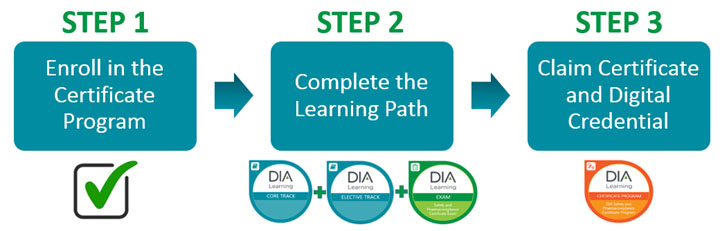
How the Program Works
DIA Safety and Pharmacovigilance Certificate Program is a competency-based program offering up to 64 Continuing Education (CE) credits, as well as up to 6.7 Continuing Education Units (CEUs). This comprehensive program is based on the DIA Safety and Pharmacovigilance Competency Framework developed with experts working in the field. These competencies outline the functional knowledge and skills needed to work in safety and pharmacovigilance and comply with US and EU regulations. The Certificate Program is designed for individuals new to the field, for those with one to three years of experience, or for those looking to broaden their expertise in this area. The purpose of the program is to provide the knowledge and skills safety professionals need to succeed in their role, as well as to provide a learning pathway that allows individuals to further develop and advance their career. The safety competencies may also be leveraged by leaders in the industry to help build safety teams and develop their employees.
Earn CE Credit While You Learn!
Enrollment in the Certificate Program is free. Registration fees apply to the specific courses you choose as part of the Learning Path. After enrolling, participants can track their progress in the Learning Path through the DIA Learning Management System (LMS). CE credit is available to participants upon completion of each module in the certificate program, at no additional cost. See individual module overview for specific CE information. DIA developed a comprehensive training curriculum and defined a Learning Path, based on this competency framework, which participants can acquire the knowledge and skills through attendance at live training courses, as well as online and blended learning courses. After a participant has completed the required 19 Core and Elective Track credits, they must pass an exam, with a score of 73% or better, designed to measure achievement of these competencies to complete their Learning Path.
Participants will earn digital credentials as they continue along the Learning Path, ultimately culminating in the granting of the DIA Safety and Pharmacovigilance Certificate represented by the digital credential. The certificate term is for life.